Crystals are nature's most orderly substances. Inside them, atoms and molecules are arranged in regular, repeating structures, giving rise to solids that are stable and rigid—and often beautiful to behold.
People have found crystals fascinating and attractive since before the dawn of modern science, often prizing them as jewels. In the 19th century scientists' quest to classify forms of crystals and understand their effect on light catalyzed important progress in mathematics and physics. Then, in the 20th century, study of the fundamental quantum mechanics of electrons in crystals led directly to modern semiconductor electronics and eventually to smartphones and the Internet.
The next step in our understanding of crystals is occurring now, thanks to a principle that arose from Albert Einstein's relativity theory: space and time are intimately connected and ultimately on the same footing. Thus, it is natural to wonder whether any objects display properties in time that are analogous to the properties of ordinary crystals in space. In exploring that question, we discovered “time crystals.” This concept, along with the growing class of novel materials that fit within it, has led to exciting insights about physics, as well as the potential for new applications, including clocks more accurate than any that exist now.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Symmetry
Before I fully explain this idea, I must clarify what, exactly, a crystal is. The most fruitful answer for scientific purposes brings in two profound concepts: symmetry and spontaneous symmetry breaking.
In common usage, “symmetry” very broadly indicates balance, harmony or even justice. In physics and mathematics, the meaning is more precise. We say that an object is symmetric or has symmetry if there are transformations that could change it but do not.
That definition might seem strange and abstract at first, so let us focus on a simple example: Consider a circle. When we rotate a circle around its center, through any angle, it remains visually the same, even though every point on it may have moved—it has perfect rotational symmetry. A square has some symmetry but less than a circle because you must rotate a square through a full 90 degrees before it regains its initial appearance. These examples show that the mathematical concept of symmetry captures an essential aspect of its common meaning while adding the virtue of precision.
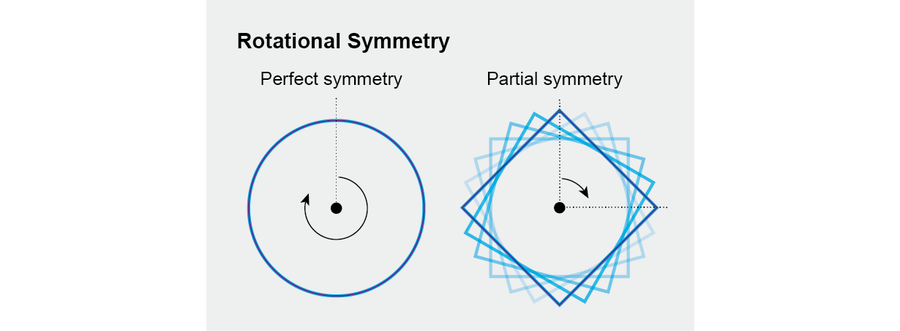
Credit: Jen Christiansen
A second virtue of this concept of symmetry is that it can be generalized. We can adapt the idea so that it applies not just to shapes but more widely to physical laws. We say a law has symmetry if we can change the context in which the law is applied without changing the law itself. For example, the basic axiom of special relativity is that the same physical laws apply when we view the world from different platforms that move at constant velocities relative to one another. Thus, relativity demands that physical laws display a kind of symmetry—namely, symmetry under the platform-changing transformations that physicists call “boosts.”
A different class of transformations is important for crystals, including time crystals. They are the very simple yet profoundly important transformations known as translations. Whereas relativity says the same laws apply for observers on moving platforms, spatial translation symmetry says the same laws apply for observers on platforms in different places. If you move—or “translate”—your laboratory from one place to another, you will find that the same laws hold in the new place. Spatial translation symmetry, in other words, asserts that the laws we discover anywhere apply everywhere.
Time translation symmetry expresses a similar idea but for time instead of space. It says the same laws we operate under now also apply for observers in the past or in the future. In other words, the laws we discover at any time apply at every time. In view of its basic importance, time translation symmetry deserves to have a less forbidding name, with fewer than seven syllables. Here I will call it tau, denoted by the Greek symbol τ.
Without space and time translation symmetry, experiments carried out in different places and at different times would not be reproducible. In their everyday work, scientists take those symmetries for granted. Indeed, science as we know it would be impossible without them. But it is important to emphasize that we can test space and time translation symmetry empirically. Specifically, we can observe behavior in distant astronomical objects. Such objects are situated, obviously, in different places, and thanks to the finite speed of light we can observe in the present how they behaved in the past. Astronomers have determined, in great detail and with high accuracy, that the same laws do in fact apply.
Symmetry Breaking
For all their aesthetic symmetry, it is actually the way crystals lack symmetry that is, for physicists, their defining characteristic.
Consider a drastically idealized crystal. It will be one-dimensional, and its atomic nuclei will be located at regular intervals along a line, separated by the distance d. (Their coordinates therefore will be nd, where n is a whole number.) If we translate this crystal to the right by a tiny distance, it will not look like the same object. Only after we translate through the specific distance d will we see the same crystal. Thus, our idealized crystal has a reduced degree of spatial translation symmetry, similarly to how a square has a reduced degree of rotational symmetry.
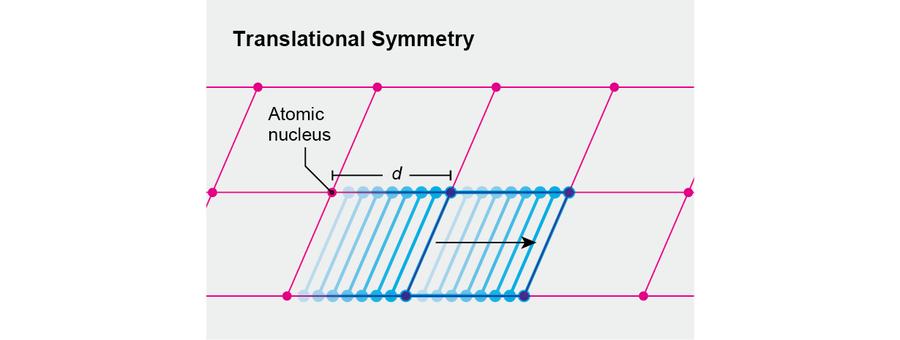
Credit: Jen Christiansen
Physicists say that in a crystal the translational symmetry of the fundamental laws is “broken,” leading to a lesser translational symmetry. That remaining symmetry conveys the essence of our crystal. Indeed, if we know that a crystal's symmetry involves translations through multiples of the distance d, then we know where to place its atoms relative to one another.
Crystalline patterns in two and three dimensions can be more complicated, and they come in many varieties. They can display partial rotational and partial translational symmetry. The 14th-century artists who decorated the Alhambra palace in Granada, Spain, discovered many possible forms of two-dimensional crystals by intuition and experimentation, and mathematicians in the 19th century classified the possible forms of three-dimensional crystals.
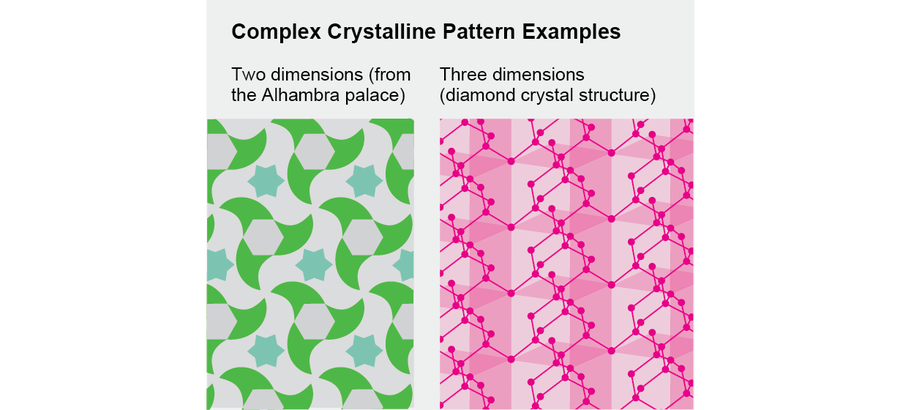
Credit: Jen Christiansen
In the summer of 2011 I was preparing to teach this elegant chapter of mathematics as part of a course on the uses of symmetry in physics. I always try to take a fresh look at material I will be teaching and, if possible, add something new. It occurred to me then that one could extend the classification of possible crystalline patterns in three-dimensional space to crystalline patterns in four-dimensional spacetime.
When I mentioned this mathematical line of investigation to Alfred Shapere, my former student turned valued colleague, who is now at the University of Kentucky, he urged me to consider two very basic physical questions. They launched me on a surprising scientific adventure:
What real-world systems could crystals in spacetime describe?
Might these patterns lead us to identify distinctive states of matter?
The answer to the first question is fairly straightforward. Whereas ordinary crystals are orderly arrangements of objects in space, spacetime crystals are orderly arrangements of events in spacetime.
As we did for ordinary crystals, we can get our bearings by considering the one-dimensional case, in which spacetime crystals simplify to purely time crystals. We are looking, then, for systems whose overall state repeats itself at regular intervals. Such systems are almost embarrassingly familiar. For example, Earth repeats its orientation in space at daily intervals, and the Earth-sun system repeats its configuration at yearly intervals. Inventors and scientists have, over many decades, developed systems that repeat their arrangements at increasingly accurate intervals for use as clocks. Pendulum and spring clocks were superseded by clocks based on vibrating (traditional) crystals, and those were eventually superseded by clocks based on vibrating atoms. Atomic clocks have achieved extraordinary accuracy, but there are important reasons to improve them further—and time crystals might help, as we will see later.
Some familiar real-world systems also embody higher-dimensional spacetime crystal patterns. For example, the pattern shown here can represent a planar sound wave, where the height of the surface indicates compression as a function of position and time. More elaborate spacetime crystal patterns might be difficult to come by in nature, but they could be interesting targets for artists and engineers—imagine a dynamic Alhambra on steroids.
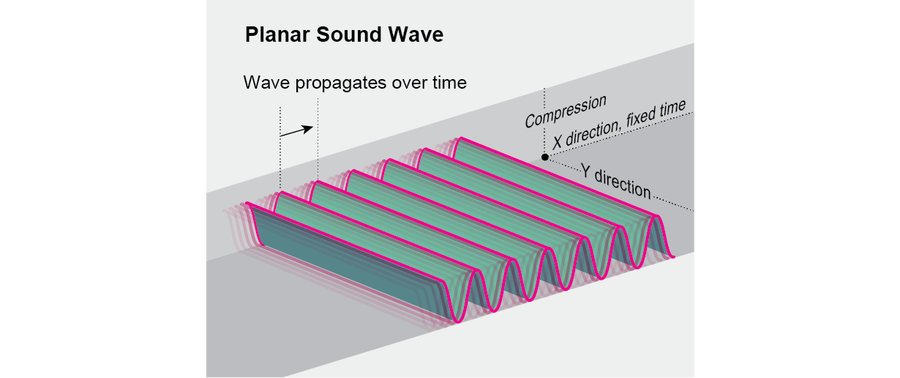
Credit: Jen Christiansen
These types of spacetime crystals, though, simply repackage known phenomena under a different label. We can move into genuinely new territory in physics by considering Shapere's second question. To do that, we must now bring in the idea of spontaneous symmetry breaking.
Spontaneous Symmetry Breaking
When a liquid or gas cools into a crystal, something fundamentally remarkable occurs: the emergent solution of the laws of physics—the crystal— displays less symmetry than the laws themselves. As this reduction of symmetry is brought on just by a decrease in temperature, without any special outside intervention, we can say that in forming a crystal the material breaks spatial translation symmetry “spontaneously.”
An important feature of crystallization is a sharp change in the system's behavior or, in technical language, a sharp phase transition. Above a certain critical temperature (which depends on the system's chemical composition and the ambient pressure), we have a liquid; below it we have a crystal—objects with quite different properties. The transition occurs predictably and is accompanied by the emission of energy (in the form of heat). The fact that a small change in ambient conditions causes a substance to reorganize into a qualitatively distinct material is no less remarkable for being, in the case of water and ice, very familiar.
The rigidity of crystals is another emergent property that distinguishes them from liquids and gases. From a microscopic perspective, rigidity arises because the organized pattern of atoms in a crystal persists over long distances and the crystal resists attempts to disrupt that pattern.
The three features of crystallization that we have just discussed—reduced symmetry, sharp phase transition and rigidity—are deeply related. The basic principle underlying all three is that atoms “want” to form patterns with favorable energy. Different choices of pattern—in the jargon, different phases—can win out under different conditions (for instance, various pressures and temperatures). When conditions change, we often see sharp phase transitions. And because pattern formation requires collective action on the part of the atoms, the winning choice will be enforced over the entire material, which will snap back into its previous state if the chosen pattern is disturbed.
Because spontaneous symmetry breaking unites such a nice package of ideas and powerful implications, I felt it was important to explore the possibility that τ can be broken spontaneously. As I was writing up this idea, I explained it to my wife, Betsy Devine: “It's like a crystal but in time.” Drawn in by my excitement, she was curious: “What are you calling it?” “Spontaneous breaking of time translation symmetry,” I said. “No way,” she countered. “Call it time crystals.” Which, naturally, I did. In 2012 I published two papers, one co-authored by Shapere, introducing the concept. A time crystal, then, is a system in which τ is spontaneously broken.
One might wonder why it took so long for the concepts of τ and spontaneous symmetry breaking to come together, given that separately they have been understood for many years. It is because τ differs from other symmetries in a crucial way that makes the question of its possible spontaneous breaking much subtler. The difference arises because of a profound theorem proved by mathematician Emmy Noether in 1915. Noether's theorem makes a connection between symmetry principles and conservation laws—it shows that for every form of symmetry, there is a corresponding quantity that is conserved. In the application relevant here, Noether's theorem states that τ is basically equivalent to the conservation of energy. Conversely, when a system breaks τ, energy is not conserved, and it ceases to be a useful characteristic of that system. (More precisely: without τ, you can no longer obtain an energylike, time-independent quantity by summing up contributions from the system's parts.)
The usual explanation for why spontaneous symmetry breaking occurs is that it can be favorable energetically. If the lowest-energy state breaks spatial symmetry and the energy of the system is conserved, then the broken symmetry state, once entered, will persist. That is how scientists account for ordinary crystallization, for example.
But that energy-based explanation will not work for τ breaking, because τ breaking removes the applicable measure of energy. This apparent difficulty put the possibility of spontaneous τ breaking, and the associated concept of time crystals, beyond the conceptual horizon of most physicists.
There is, however, a more general road to spontaneous symmetry breaking, which also applies to τ breaking. Rather than spontaneously reorganizing to a lower-energy state, a material might reorganize to a state that is more stable for other reasons. For instance, ordered patterns that extend over large stretches of space or time and involve many particles are difficult to unravel because most disrupting forces act on small, local scales. Thus, a material might achieve greater stability by taking on a new pattern that occurs over a larger scale than in its previous state.
Ultimately, of course, no ordinary state of matter can maintain itself against all disruptions. Consider, for example, diamonds. A legendary ad campaign popularized the slogan “a diamond is forever.” But in the right atmosphere, if the temperature is hot enough, a diamond will burn into inglorious ash. More basically, diamonds are not a stable state of carbon at ordinary temperatures and atmospheric pressure. They are created at much higher pressures and, once formed, will survive for a very long time at ordinary pressures. But physicists calculate that if you wait long enough, your diamond will turn into graphite. Even less likely, but still possible, a quantum fluctuation can turn your diamond into a tiny black hole. It is also possible that the decay of a diamond's protons will slowly erode it. In practice, what we mean by a “state of matter” (such as diamond) is an organization of a substance that has a useful degree of stability against a significant range of external changes.
Old and New Time Crystals
The AC Josephson effect is one of the gems of physics, and it supplies the prototype for one large family of time crystals. It occurs when we apply a constant voltage V (a difference in potential energy) across an insulating junction separating two superconducting materials (a so-called Josephson junction, named after physicist Brian Josephson). In this situation, one observes that an alternating current at frequency 2eV/ℏ flows across the junction, where e is the charge of an electron and ℏ is the reduced Planck's constant. Here, although the physical setup does not vary in time (in other words, it respects τ), the resulting behavior does vary in time. Full time translation symmetry has been reduced to symmetry under time translation by multiples of the period ℏ/2eV. Thus, the AC Josephson effect embodies the most basic concept of a time crystal. In some respects, however, it is not ideal. To maintain the voltage, one must somehow close the circuit and supply a battery. But AC circuits tend to dissipate heat, and batteries run down. Moreover, oscillating currents tend to radiate electromagnetic waves. For all these reasons, Josephson junctions are not ideally stable.
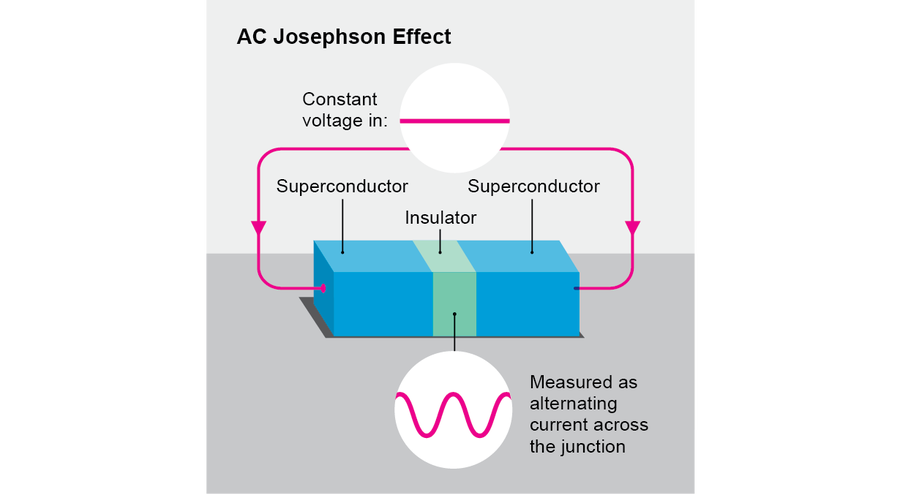
Credit: Jen Christiansen
By using refinements such as fully superconducting circuits, excellent capacitors in place of ordinary batteries and enclosures to trap radiation, it is possible to substantially reduce the levels of those effects. And other systems that involve superfluids or magnets in place of superconductors exhibit analogous effects while minimizing those problems. Nikolay Prokof'ev and Boris Svistunov have proposed extremely clean examples involving two interpenetrating superfluids.
Thinking explicitly about τ breaking has focused attention on these issues and led to the discovery of new examples and fruitful experiments. Still, because the central physical idea is already implicit in Josephson's work of 1962, it seems appropriate to refer to all these as “old” time crystals.
“New” time crystals arrived with the March 9, 2017, issue of Nature, which featured gorgeous (metaphorical) time crystals on the cover and announced “Time crystals: First observations of exotic new state of matter.” Inside were two independent discovery papers. In one experiment, a group led by Christopher Monroe of the University of Maryland, College Park, created a time crystal in an engineered system of a chain of ytterbium ions. In the other, Mikhail Lukin's group at Harvard University realized a time crystal in a system of many thousands of defects, called nitrogen vacancy centers, in a diamond.
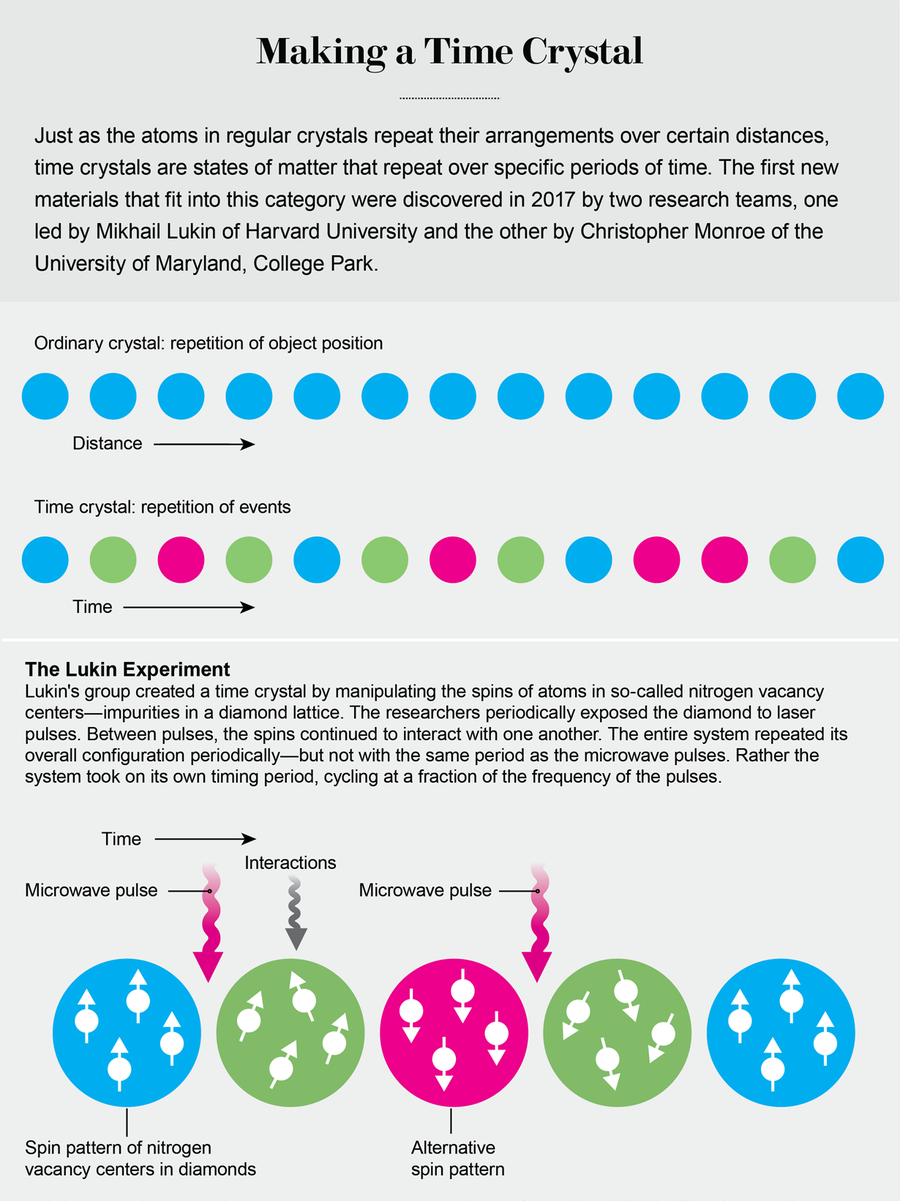
Credit: Jen Christiansen
In both systems, the spin direction of the atoms (either the ytterbium ions or the diamond defects) changes with regularity, and the atoms periodically come back into their original configurations. In Monroe's experiment, researchers used lasers to flip the ions' spins and to correlate the spins into connected, “entangled” states. As a result, though, the ions' spins began to oscillate at only half the rate of the laser pulses. In Lukin's project, the scientists used microwave pulses to flip the diamond defects' spins. They observed time crystals with twice and three times the pulse spacing. In all these experiments, the materials received external stimulation—lasers or microwave pulses—but they displayed a different period than that of their stimuli. In other words, they broke time symmetry spontaneously.
These experiments inaugurated a direction in materials physics that has grown into a minor industry. More materials utilizing the same general principles—which have come to be called Floquet time crystals—have come on the scene since then, and many more are being investigated.
Floquet time crystals are distinct in important ways from related phenomena discovered much earlier. Notably, in 1831 Michael Faraday found that when he shook a pool of mercury vertically with period T, the resulting flow often displayed period 2T. But the symmetry breaking in Faraday's system—and in many other systems studied in the intervening years prior to 2017—does not allow a clean separation between the material and the drive (in this case, the act of shaking), and it does not display the hallmarks of spontaneous symmetry breaking. The drive never ceases to pump energy (or, more accurately, entropy), which is radiated as heat, into the material.
In effect, the entire system consisting of material plus drive—whose behavior, as noted, cannot be cleanly separated—simply has less symmetry than the drive considered separately. In the 2017 systems, in contrast, after a brief settling-down period, the material falls into a steady state in which it no longer exchanges energy or entropy with the drive. The difference is subtle but physically crucial. The new Floquet time crystals represent distinct phases of matter, and they display the hallmarks of spontaneous symmetry breaking, whereas the earlier examples, though extremely interesting in their own right, do not.
Likewise, Earth's rotation and its revolution around the sun are not time crystals in this sense. Their impressive degree of stability is enforced by the approximate conservation of energy and angular momentum. They do not have the lowest possible values of those quantities, so the preceding energetic argument for stability does not apply; they also do not involve long-range patterns. But precisely because of the enormous value of energy and angular momentum in these systems, it takes either a big disturbance or small disturbances acting over a long time to significantly change them. Indeed, effects that include the tides, the gravitational influence of other planets and even the evolution of the sun do slightly alter those astronomical systems. The associated measures of time such as “day” and “year” are, notoriously, subject to occasional correction.
In contrast, these new time crystals display strong rigidity and stability in their patterns—a feature that offers a way of dividing up time very accurately, which could be the key to advanced clocks. Modern atomic clocks are marvels of accuracy, but they lack the guaranteed long-term stability of time crystals. More accurate, less cumbersome clocks based on these emerging states of matter could empower exquisite measurements of distances and times, with applications from improved GPS to new ways of detecting underground caves and mineral deposits through their influence on gravity or even gravitational waves. DARPA—the Defense Advanced Research Projects Agency—has funded research on time crystals with such possibilities in mind.
THE TAO OF τ
The circle of ideas and experiments around time crystals and spontaneous τ breaking represents a subject in its infancy. There are many open questions and fronts for growth. One ongoing task is to expand the census of physical time crystals to include larger and more convenient examples and to embody a wider variety of spacetime patterns, by both designing new time crystal materials and discovering them in nature. Physicists are also interested in studying and understanding the phase transitions that bring matter into and out of these states.
Another task is to examine in detail the physical properties of time crystals (and spacetime crystals, in which space symmetry and τ are both spontaneously broken). Here the example of semiconductor crystals, mentioned earlier, is inspiring. What discoveries will emerge as we study how time crystals modify the behavior of electrons and light moving within them?
Having opened our minds to the possibility of states of matter that involve time, we can consider not only time crystals but also time quasicrystals (materials that are very ordered yet lack repeating patterns), time liquids (materials in which the density of events in time is constant but the period is not) and time glasses (which have a pattern that looks perfectly rigid but actually shows small deviations). Researchers are actively exploring these and other possibilities. Indeed, some forms of time quasicrystals and a kind of time liquid have been identified already.
So far we have considered phases of matter that put τ into play. Let me conclude with two brief comments about τ in cosmology and in black holes.
The steady-state-universe model was a principled attempt to maintain τ in cosmology. In that model, popular in the mid-20th century, astronomers postulated that the state, or appearance, of the universe on large scales is independent of time—in other words, it upholds time symmetry. Although the universe is always expanding, the steady-state model postulated that matter is continuously being created, allowing the average density of the cosmos to stay constant. But the steady-state model did not survive the test of time. Instead astronomers have accumulated overwhelming evidence that the universe was a very different place 13.7 billion years ago, in the immediate aftermath of the big bang, even though the same physical laws applied. In that sense, τ is (perhaps spontaneously) broken by the universe as a whole. Some cosmologists have also suggested that ours is a cyclic universe or that the universe went through a phase of rapid oscillation. These speculations—which, to date, remain just that—bring us close to the circle of ideas around time crystals.
Finally, the equations of general relativity, which embody our best present understanding of spacetime structure, are based on the concept that we can specify a definite distance between any two nearby points. This simple idea, though, is known to break down in at least two extreme conditions: when we extrapolate big bang cosmology to its initial moments and in the central interior of black holes. Elsewhere in physics, breakdown of the equations that describe behavior in a given state of matter is often a signal that the system will undergo a phase transition. Could it be that spacetime itself, under extreme conditions of high pressure, high temperature or rapid change, abandons τ?
Ultimately the concept of time crystals offers a chance for progress both theoretically—in terms of understanding cosmology and black holes from another perspective—and practically. The novel forms of time crystals most likely to be revealed in the coming years should move us closer to more perfect clocks, and they may turn out to have other useful properties. In any case, they are simply interesting, and offer us opportunities to expand our ideas about how matter can be organized.

