Göran Gustafsson looks at people and thinks of cars—the ageing models that rolled off assembly lines a few decades ago. Today, says Gustafsson, cars are packed with cutting-edge sensors, computers and sophisticated communications systems that warn of problems when they are still easy to fix, which is why modern vehicles rarely surprise their drivers with catastrophic breakdowns.
“Why don't we have a similar vision for our bodies?” wonders Gustafsson, an engineer whose team at the Swedish electronics company Acreo, based in Kista, is one of many around the world trying to make such a vision possible. Instead of letting health problems go undetected until a person ends up in hospital—the medical equivalent of a roadside breakdown—these teams foresee a future in which humans are wired up like cars, with sensors that form a similar early-warning system.
Working with researchers at Linköping University in Sweden, Gustafsson's team has developed skin-surface and implanted sensors, as well as an in-body intranet that can link devices while keeping them private. Other groups are developing technologies ranging from skin patches that sense arterial stiffening—a signal of a looming heart attack—to devices that detect epileptic fits and automatically deliver drugs directly to affected areas of the brain.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
These next-generation devices are designed to function alongside tissue, rather than be isolated from it like most pacemakers and other electronic devices already used in the body. But making this integration work is no easy feat, especially for materials scientists, who must shrink circuits radically, make flexible and stretchable electronics that are imperceptible to tissue, and find innovative ways to create interfaces with the body. Achieving Gustafsson's vision—in which devices monitor and treat the body day in, day out—will also require both new power sources and new ways of transmitting information.
Still, the potential to improve health care substantially while reducing its costs has drawn both researchers and physicians to the challenge, says John Rogers, a materials scientist at the University of Illinois at Urbana–Champaign. “I haven't found any clinical folks who say 'That's pie in the sky, come back to me in 20 years,'” he says. “They say, 'Wow, that's cool. Here are three ways we can use it today, and how do we get started on a collaboration?'.”
Sensors woven into the body are a natural extension of handheld smartphones and wearable devices, says Rogers. “I think electronics is coming at you,” he says. “It's migrating closer and closer and I think it's a very natural thing to imagine that they will eventually become intimately integrated with the body.”
Skin deep
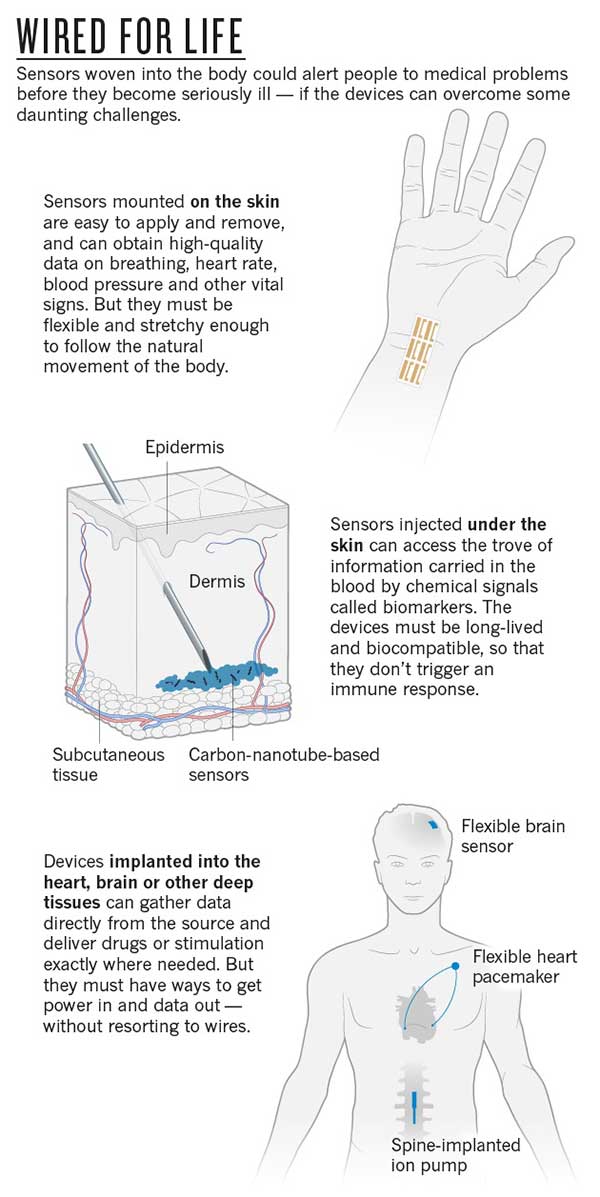
The first step beyond wearables will be wireless sensors mounted directly on the skin, where they can pick up a host of vital signs, including temperature, pulse and breathing rate. Unfortunately, says Rogers, “biology involves bending, stretching and swelling”, which makes conventional electronics built from stiff silicon wafers a very poor choice for such sensors.
His team has developed 'epidermal electronics': flexible, biodegradable stick-on patches that are crammed with sensors but almost imperceptible to the user. Attached like temporary tattoos, the patches use normal silicon electronics, but thinned down and transferred to a flexible backing using a rubber stamp. The patches draw power either from nearby magnetic fields or by harvesting radio waves, using S-shaped wires and antennas designed to stretch, twist and bend. “They adopt a wavy kind of geometry, so when you stretch, the wave shapes can change, like accordion bellows,” says Rogers.
Rogers has co-founded a spin-off company—MC10, based in Lexington, Massachusetts—that next year will start marketing versions of the device as BioStamps: temporary patches that measure heart electrical activity, hydration, body temperature and exposure to ultraviolet light. The patches will be available to consumers first, says Rogers, but his real target is medicine. Results are expected soon from a trial at the neonatal intensive-care unit at Carle Foundation Hospital in Urbana, where doctors are using the patches to monitor the vital signs of newborn babies without the need for intrusive cables and scanners. MC10 is also collaborating with Brussels-based pharmaceutical company UCB on tests of a patch that monitors tremors in people with Parkinson's disease, to track their illness and whether they are taking their medication.
Rogers' patches are relatively small, but at the University of Tokyo, engineer Takao Someya has created a sensor-laden electronic skin that can be made in much larger pieces. His latest film is just 1 micrometre thick, and so light that it floats like a feather, yet it is robust enough to cope with the stretching and crumpling needed to flex with an elbow or knee. It can provide readouts on temperature—heat in a wound can signal infection—moisture, pulse and oxygen concentration in the blood. Someya achieves this by ditching silicon altogether, and instead using inherently soft organic components made of carbon-based polymers and other materials. Organic circuits can be printed onto a plastic film, making them cheap and easy to produce in large quantities. And they are versatile: they work in both high-temperature and water-based environments.
Skin also inspires Zhenan Bao, an engineer at Stanford University in California. Her team creates thin pressure sensors by sandwiching micrometre-scale rubber pyramids between films. Even a slight touch will compress the pyramids' tips, changing how electric current flows between the films. The sensors can be used in heart monitors that track how fast pressure waves pass through arteries. This can reveal increased stiffness in the vessels—a predictor of heart attacks. Last year, the US Food and Drug Administration approved a wireless pressure sensor that can be implanted inside the hearts of people with advanced heart disease; Bao's device could do a similar job from the surface of the skin.
Credit: Nature
As useful as skin-mounted patches might be, much more information is available deeper in the body. “There's a reason why at the hospital, they draw your blood,” says Michael Strano, a chemical engineer at the Massachusetts Institute of Technology (MIT) in Cambridge. “There are markers in blood that are exquisitely good at predicting disease.”
But delving deeper brings fresh challenges. Ideally, says Strano, sensors under the skin should be not only non-toxic, but also stable enough to function inside the body for years at a time if need be, and biocompatible—meaning that they don't trigger the body's immune response. Yet most current devices fall short on one score or another. For example, sensors that detect chemical signals in the blood called biomarkers often use biological materials that degrade very quickly. This is a severe limitation for the advanced, real-time sensors that are currently used to monitor glucose in people with diabetes, says Strano: the devices detect glucose with an enzyme reaction that produces hydrogen peroxide. This degrades the sensors so quickly that they must be replaced within weeks.
To get around that, Strano's lab has developed synthetic, long-lived detector materials that can be mixed with a water-based gel and injected under the skin like a tattoo. The 'ink' for this tattoo consists of carbon nanotubes coated with dangling polymer strands, which have a lock-and-key chemical structure that recognizes biomarkers by dictating which molecules can dock with them. When biomarkers bind to the polymer, they subtly change the optical properties of the nanotube: shine a light on the tattoo, and a glow reveals the presence of the biomarker.
Strano and his team have developed carbon-nanotube sensors to monitor nitric oxide in blood—an inflammatory marker that can indicate infection or even cancer—and are working on glucose and cortisol, a stress biomarker that may prove useful for monitoring post-traumatic stress disorder and anxiety disorders. The nitric oxide sensor worked for 400 days in mice, which to Strano's knowledge is the longest any implanted chemical sensor has been in place, and did so without provoking any immune response. For many other kinds of device, the jury is still out. “For electronic materials, especially plastic-based and organics, it's still unknown what their long-term effects are,” says Bao.
Now Strano is starting work with MIT engineer Daniel Anderson on devices that could combine sensors with drug-delivery systems. They hope to adapt microchips pioneered by fellow MIT engineer Robert Langer to respond to a range of triggers by releasing the appropriate drugs, encased in polymer capsules. The first human trial of a drug-delivering 'pharmacy on a chip'—without the sensors—was in 2012, in eight women with osteoporosis.
It will be a long time before such devices can be used to detect diseases reliably and treat them automatically, except perhaps for diabetes, which has been extensively studied. Strano's devices are good at binding only with their target molecules, but big questions remain about what fluctuations in biomarker signals actually mean in terms of health, he says. His team is modelling biomarkers in the body, to help to decide where the sensor needs to be and how quickly it should to react to give useful information. “Often you need to rely on many different sensory parameters to make a decision. It's not enough that one chemical is over-expressed,” says Magnus Berggren, an electronic engineer at Linköping University who is collaborating with Gustafsson.
Moving target
Some researchers' targets lie still deeper in the body, and for them, flexibility and biocompatibility are even more important. If a rigid sensor rubs against a moving organ such as the heart or the brain, in which the cells shift slight as the animal breathes, the body will quickly surround it with a wall of scar tissue. And if sensors move relative to the organ, the results will be unreliable in any case.
Bioelectronics engineer George Malliaras at the École Nationale Supérieure des Mines de Saint-Étienne in Gardanne, France, and his colleagues are among those developing flexible replacements for the relatively rigid sensors currently used to track distinctive electrical patterns in the brains of people with epilepsy or Parkinson's disease. Made of organic, conducting polymers, these flexible electronics respond to chemical signals—the flow of ions that generates the electrical patterns. This not only increases sensitivity, but also lets researchers “interface with biology in a wholly different fashion”, he says.
The team's latest device, tested in rats as well as in two humans undergoing surgery for epilepsy, has detected the firing of individual neurons, says Malliaras. And if the process is reversed, he adds, the sensor can be used to deliver drugs. Devices known as organic electronic ion pumps respond to an applied voltage by forcing drugs—small charged particles—out of a reservoir. Working with the group at Linköping University and the French National Institute of Health and Medical Research in Marseilles, Malliaras's team is coupling his epilepsy sensor to an ion pump that responds to seizures by releasing epilepsy drugs into the correct part of the brain. Berggren and the Linköping team have used a similar technique to develop a 'pacemaker for pain' that delivers analgesics directly to the spinal cord.
Keep it going
Any electrical device is limited by its need for power. Devices that sit on or near the skin can incorporate antennas that harvest power wirelessly—as long as an external source is nearby. But sensors deeper in the body often have to rely on batteries, which are bulky and need replacing. And some, such as Berggren's pain-relief pump, need to have wires threaded through the overlying tissues—an arrangement that is both cumbersome and a potential route for infection (see 'Wired for life').
To get around such problems, Zhong Lin Wang, a nanoscientist at the Georgia Institute of Technology in Atlanta, has spent the past decade trying to harvest the tiny amounts of mechanical energy generated when people walk or even breathe. “We started thinking, how do we convert body motion into electricity?” he says.
His latest design uses static electricity—long thought of as a nuisance—to convert the movement of inhaling and exhaling into enough energy to power a pacemaker. The generator uses two different polymer surfaces, sandwiched between electrodes and connected in a circuit. When the user breathes in and out, the surfaces touch and separate, swapping electrons—the same thing that happens when a balloon is stroked with a wool cloth. The build-up of charge causes current to flow through the wire. “Inhale and exhale, move back and forth or drive up and down and you generate power,” says Wang.
Starting in 2014, Wang began testing the system in rats, creating milliwatts of energy from a device the thickness of a few sheets of paper. Now his team is testing the same technology in pigs.
Rogers' team has created a biodegradable battery using electrodes made of magnesium and other metals that are safe in low concentrations and that slowly dissolve in the body. “Some devices you want to last the life of the patient. In others, you only need and want the device to be temporary,” says Rogers.
Personal privacy
The technology could be revolutionary, but the vision of a wired-up body that sends data to an outside computer or medical centre faces a threat that already troubles the wearables industry:hacking. “When a semiconductor chip is introduced inside the body, hacking is a truly serious issue,” says Someya.
One solution is to analyse data on the device itself, reducing the amount that gets sent over the airwaves. Another is to avoid the airwaves altogether. In as-yet-unpublished work, the Swedish team has developed an in-body intranet that transmits signals at low frequency using the body's water as its wires. To send information between devices, or from a device to a smartphone, users must physically touch the objects with their hands. This keeps the signals low-power and private, and avoids clogging up the data-transmitting frequencies that are already squabbled over by mobile phones and wireless routers. “It's only transmitted and exposed within your body,” adds Berggren, who says that the system can already exchange data between electronically labelled objects through the body to a smartphone, and will soon integrate on-skin sensors.
However good the devices, pioneers of new materials will also struggle against a tide of medical regulation, says Malliaras. That, along with the concerns of chemical suppliers who are afraid that failing devices could leave them vulnerable to lawsuits, “puts a big brake on the adoption of new materials”, he says.
Berggren and his collaborators at Acreo are among the first to try to connect a range of devices by wiring up humans. But they readily acknowledge that making the vision a reality will require multiple companies and research teams, as well as the involvement of insurance companies and health-care providers.
Berggren knows that there are big hurdles. “The challenge is to put everything together,” he says. “But they did it for the car industry and it's impressive. You rarely see cars standing along the side of the road waiting for repair. Whether it's possible to do this also for humans is still a question mark, but it's definitely worth trying.”
Malliaras agrees. “A car you usually keep for less than ten years,” he says. “A body you want to keep for 80 or 90 years; it's a lot more precious.”
This article is reproduced with permission and was first published on December 1, 2015.